UPDATES
Their findings have been published in the journal ACS Nano on October 14, 2020.
An international team of researchers, led by Distinguished Professor Rodney S. Ruoff (Department of Chemistry) from the Center for Multidimensional Carbon Materials (CMCM), within the Institute for Basic Science (IBS) at UNIST, has synthesized a film composed of densely packed diamond-like carbon nanofibers. As described in a recent article published in the journal ACS Nano, the researchers noted that the new carbon material has a high concentration of tetravalently-bonded carbons (the diamond-like nanofibers have many C atoms with 4 other atoms bonded to them; this is also referred to as “sp3-bonded carbon”).
“The diamond-like carbon nanofiber films were synthesized by heating copper nanoparticles of few nanometers in diameter on a substrate, in acetylene and hydrogen gases,” says Kee Han Lee. “The synthesized fibers were highly dense and formed a film. These densely packed nanofibers could also be separated into a powder form which could potentially broaden their applications.”
In this study, the team was able to identify parameters influencing the packing density of the nanofibers namely the hydrogen gas concentration and the copper nanoparticle catalyst size. The packing density could be significantly increased by adjusting these parameters, which led to the formation of a buckled film.
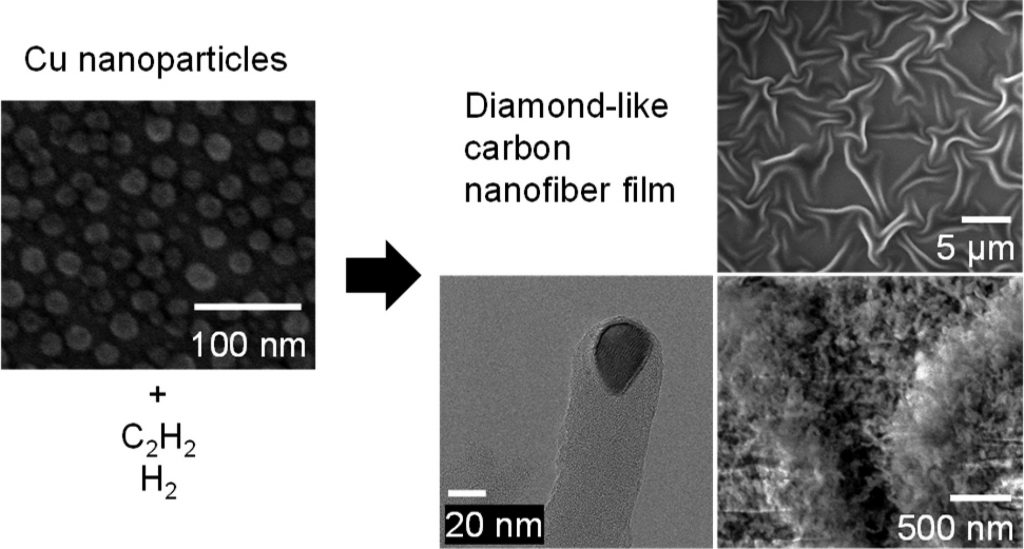
Figure. Field Emission-Scanning Electron Microscope images of copper nanoparticles and of the synthesized diamond-like carbon fiber film, and a High-Resolution transmission electron microscope image of a single nanofiber.
“With its high sp3-carbon content, this material is different from other vapor grown carbon nanofibers including nanotubes, which are mostly composed of stacked graphene layers, and carbon nanocoils that have an sp3: sp2 carbon ratio of about 0.25,” explains Sun Hwa Lee.
The electrical resistivity (1.2 ± 0.1 × 106 Ω cm—it is electrically insulating), density (2.5 ± 0.2 g cm-3; the density of diamond is 3.5 g cm-3), surface area (28 ± 0.7 m2 g-1), chemical inertness, and wettability towards various liquids, were determined. These properties were similar or “better” than most diamond-like carbon films reported, but the mechanical properties of this diamond-like carbon nanofiber film were found to be entirely different than conventional, continuous, diamond-like carbon films, because it is composed of nanofibers.
“We discovered a new form of carbon, and one might expect our work to inspire others to now also study further along this research path,” said Distinguished Professor Ruoff. “We do basic science of new carbon materials among other things, and we are interested in eventually achieving pure diamond fibers, along with further studies of this type of diamond-like carbon nanofiber.”
News Article Credit to UNIST; Click HERE to see the original post
