UPDATES
IBS researchers disprove the idea that water degrades black phosphorus and find the materials is actually hydrophobic
Researchers at the Center for Multidimensional Carbon Materials (CMCM), within the Institute for Basic Science (IBS) have discovered that one of graphene's competitors, black phosphorus, is inert to water deprived of oxygen, ending the debate of whether water causes its degradation. Their research, accepted by Chemistry of Materials, provides a more complete understanding of the role of molecular oxygen and water in the degradation of black phosphorus.
Black phosphorus is a 2D materials structurlly similar to graphene with extraordinary electrical and optoelectric properties. However, unlike graphene, black phosphorus has the advantage of having a tunable bandgap. A bandgap is an energy barrier, essential for controlling the flow of electrons, like an on/off switch. Black phosphorus' bandgap varies depending on the number of black phosphorus layers: The more layers, the smaller the bandgap. This makes it interesting for the next generation nanoelectronic and photoelectronic devices. However, the perceived instability of black phosphorus to oxygen and water was never before been carefully addressed.
The IBS team questioned the previous thinking on the degradation mechanisms of black phosphorous. Researchers tested samples of black phosphorous under a number of different conditions. They checked if there is a difference between water that contains air and de-aerated water. They found that the physical and electronic properties of samples stored in de-aerated water did not degrade.
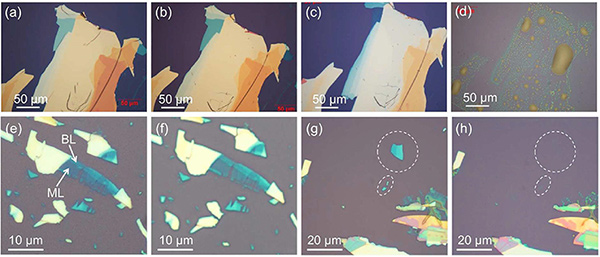
▲Effects of air and water exposure on black phosphorus.
(top) Black phosphorus flakes degraded after one week (b) and two weeks (c) of immersion in de-aerated water, but they comletely dissolved after one week exposure to air leaving behind some drop-shaped residues(d).
(bottom) Two days in de-aerated water leaves the flakes untouched (f), but 'normal' water (containing oxygen dissolved in it) is the cause of degradation. ML and BL mean monolayer and bilayer graphene.
To further clarify the role of oxygen in the degradation of black phosphorus, IBS researchers performed experiments with different oxygen isotopes (oxygen-18 and oxygen-16). They used gas with oxygen-18 and water with oxygen-16, so they could distinguish if the damage was caused by oxygen, water or both. The results confirmed that it is not water, but rather oxygen, that reacts with black phosphorous.
Furthermore, IBS researchers idscovered that the surface of black phosphorous is actually hydrophobic, in contrast to previous experiments. "It was previously thought that water could react with black phosphorous, but thanks to these experiments, we can be reassured that it is oxygen, not water, that damages black phosphorous," notes Prof. Rodney Ruoff, CMCM director and corresponding author.
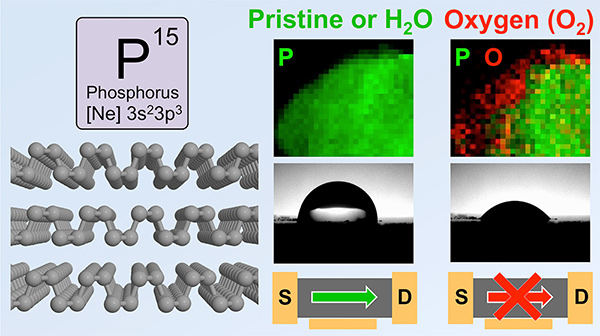
▲(Left) The layered molecular strucure of black phosphorous.
(Top Right) Scanning transmission electron microscope of a black phosphorous flake immediately before and after exposure to air for one day. Oxygen atoms (red) are detected at the edge of the flake after air exposure.
(Bottom Right) A drop of water applied on the surface of freshly cleaved black phosphorous shows that the material is hydrophobic, but it becomes hydrophillic when oxidized in air. Black phosphorous devices are stable in water, but degradatio in O2 lowers their performance.
"Water by itself does not cause any damage because it is just absorbed by the surface of black phosphorous flakes as an intact molecule. Oxyen, instead, dissociates into two oxygen atoms and oxidizes the flake. Once the materials is oxidizes by oxygen, water absorbs stronger, transforming the material from hydrophobic to hydrophilic," explains Ruoff.
In summary, water (deprived of oxygen) causes only small changes due to the fact that it is difficult to get rid of all oxygen in our environment, but oxygen substantially modifies the electronic structure of this 2D material and accelerates its degradation.
These results could open new pathways for exploring applications that require contact with aqueous solutions such as" Solution gating; electrochemistry; and solution-phase approaches for exfoliation, dispersion, and delivery of black phosphorus.
Most of the experiments were performed by Dr. Yuan Huang (CMCM, IBS), in collaboration with colleagues at a number of institutitons, including Prof. Peter Sutter (Universiy of Nebraska).
Letizia Diamante
Notes for editors
-References
Yuan Huang, Jingsi Qiao, Kai He, Stoyan Bliznakov, Eli Sutter, Xianjue Chen, Da Luo, Fanke Meng, Dong Su, Jeremy Decker, Wei Ji, Rodney S. Ruoff, and Peter Sutter. Interaction of Black Phosphorus with Oxygen and Water. Chemistry of Materials (2016). DOI: 10.1021/acs.chemmater:6b03592
