UPDATES
-IBS researchers report fundamental study of how graphene is hydrogenated-
Adding hydrogen
to graphene could improve its future applicability in the semiconductor
industry, when silicon leaves off. Researchers at the Center for Multidimensional
Carbon Materials (CMCM),
within the Institute for Basic Science (IBS) have recently gained
further insight into this chemical reaction. Published in Journal of the
American Chemical Society, these findings extend the knowledge of the
fundamental chemistry of graphene and bring scientists perhaps closer to
realizing new graphene-based materials.
Understanding how graphene can
chemically react with a variety of chemicals will increase its utility. Indeed,
graphene has superior conductivity properties, but it cannot be directly used
as an alternative to silicon in semiconductor electronics because it does not
have a bandgap, that is, its electrons can move without climbing any energy
barrier. Hydrogenation of graphene opens a bandgap in graphene, so that it
might serve as a semiconductor component in new devices.
While other reports describe the
hydrogenation of bulk materials, this study focuses on hydrogenation of single
and few-layers thick graphene. IBS scientists used a reaction based on lithium
dissolved in ammonia, called the "Birch-type reaction", to introduce
hydrogen onto graphene through the formation of C-H bonds.
The research team discovered that hydrogenation proceeds rapidly over the entire surface of single-layer graphene, while it proceeds slowly and from the edges in few-layer graphene. They also showed that defects or edges are actually necessary for the reaction to occur under the conditions used, because pristine graphene with the edges covered in gold does not undergo hydrogenation.
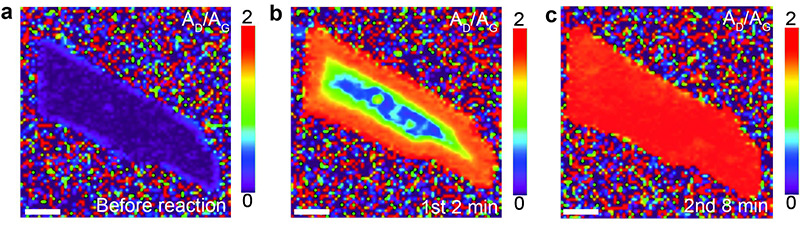
▲ Hydrogenation (in red) of bilayer graphene via Birch-type
reaction begins from the edges.
The images show a graphene flake before (a), two minutes (b), and
eight minutes (c), after exposure to a solution of lithium and liquid
ammonia (Birch-type reaction).
Graphene gets gradually hydrogenated starting from the edges. (Reprinted with
permission from Zhang X et al, JACS, Copyright 2016
American Chemical Society)
Using bilayer and trilayer graphene, IBS scientists also discovered that the reagents can pass between the layers, and hydrogenate each layer equally well. Finally, the scientists found that the hydrogenation significantly changed the optical and electric properties of the graphene.
"A primary goal of our Center is to undertake fundamental studies about reactions involving carbon materials. By building a deep understanding of the chemistry of single-layer graphene and a few layer graphene, I am confident that many new applications of chemically functionalized graphenes could be possible, in electronics, photonics, optoelectronics, sensors, composites, and other areas," notes Rodney Ruoff, corresponding author of this paper, CMCM director, and UNIST Distinguished Professor at the Ulsan National Institute of Science and Technology (UNIST).
Letizia Diamante
Notes for editors
- References
Xu Zhang, Yuan Huang, Shanshan Chen, Na Yeon Kim, Wontaek Kim,
David Schilter, Mandakini Biswal, Baowen Li, Zonghoon Lee, Sunmin Ryu,
Christopher W. Bielawski, Wolfgang S. Bacsa & Rodney S. Ruoff. Birch-type
hydrogenation of few-layer graphenes: products and mechanistic implications. J.
Am. Chem. Soc. (2016), DOI: 10.1021/jacs.6b08625
- Media Contact
For further information or to request media assistance, please
contact: Mr. Shi Bo Shim, Head of Department of Communications, Institute for
Basic Science (+82-42-878-8189, sibo@ibs.re.kr); Ms. Carol Kim, Global Officer,
Department of Communications, Institute for Basic Science (+82-42-878-8133,
clitie620@ibs.re.kr) or Letizia Diamante, Science Writer and Visual Producer
(+82-42-878-8260, letizia@ibs.re.kr)
- About the Institute for Basic Science (IBS)
IBS was founded in 2011
by the government of the Republic of Korea with the sole purpose of driving
forward the development of basic science in South Korea It comprises a total of
50 research centers in all fields of basic science, including mathematics,
physics, chemistry, life science, earth science and interdisciplinary science.
IBS has launched 26 research centers as of September 2016. There are eight
physics, one mathematics, six chemistry, eight life science, and three
interdisciplinary research centers.
-[Azonano] IBS Researchers Study Hydrogenation of Graphene
-[Engineering materials] Better graphene through chemistry
-[Science Daily] Adding hydrogen to graphene
-[Electronics360] Is Graphene Finally A Replacement for Silicon?
-[Phys.org] Adding hydrogen to graphene
-[Alpha Galileo] Adding Hydrogen to Graphene
-[New Electronics] Hydrogenation of graphene could enable new applications
-[Innovations Report] Adding hydrogen to graphene
-[Nanowerk] Adding hydrogen to graphene improves applicability in the semiconductor industry
-[Solid State Technology] Adding hydrogen to graphene
-[e! Science News] Adding
hydrogen to graphene
