UPDATES
-Understanding the interactions between Gallium and Hydrogen-
The Materials group at the Center for Multidimensional Carbon Materials directed by UNIST Distinguished Professor Rodney S. Ruoff, within the Institute for Basic Science (IBS), have discovered a large transfer of charge from metal atoms to both hydrogen molecule and hydrogen atoms – a result with potential significance in utilizing hydrogen as clean energy source, as well as in chemical synthesis. These findings were based on their observations from computer simulations of a hydrogen – liquid gallium system held at 100 °C.
Metals are materials that can conduct electricity and heat. They are commonly thought of as shiny solids, but can also exist as liquids. A well-known example of a liquid metal is mercury. Liquid metals find a wide variety of potential uses. For example, there are a growing number of studies of their use as reaction solvents and in catalysis. Among liquid metals, gallium (Ga) is of special interest, as it has a near room temperature melting point (29.76 °C), a wide liquid-state temperature range (its boiling point is 2204 °C), and low toxicity.
The growing demand for clean and renewable power sources has led to a resurgence of studies about hydrogen-based fuel cells. Hydrogen is the most abundant element in the universe, but pure molecular hydrogen (H2) gas is relatively rare. The current typical method to purify hydrogen involves the use of platinum, an expensive metal, operating at high temperatures. Recently an alternative method was demonstrated by passing the gas through a liquid gallium membrane at significantly lower temperatures. While this result was promising, the mechanism of how the hydrogen moves through the liquid gallium remains unknown. For example, does it diffuse through as H2 or does the molecular hydrogen dissociate into H atoms that then diffuse through the liquid gallium. No experiment has addressed this issue, yet.
In their simulations of hydrogen atoms dissolved in liquid gallium (done by Research Fellow Dr. Dulce Camacho-Mojica), local arrangements of Ga and H atoms within the liquid were similar in structure to the molecule of digallane (Ga2H6; see Figure 1 below) suggesting “molecular-type” bonding occurs when hydrogen atoms are present in liquid gallium. The same type of “three center ‘bridge’ bonds” that are present in the digallane molecule (Figure 1) were also found in small clusters of Ga and H atoms, and the authors discovered from their simulations that there is significant charge transfer between the liquid Ga atoms that are near the hydrogen atoms—as they also found from their theoretical study of the digallane molecule.
The authors also found that points of highest electronic density were located around the hydrogen atoms, again just like in the digallane molecule. In addition to the delocalized metallic bonding commonly seen in liquid metals, surprisingly the authors were able to distinguish highly localized molecular bonding between atoms (see Figure 2). First author of the paper, Dulce Camacho-Mojica explained how this occurred. “When hydrogen atoms enter the liquid gallium system, they gain a partial negative charge by taking electrons from neighboring gallium atoms. This is interesting as H is most often seen giving up its electron (in many other systems), rather than accepting an electron. These hydride ions (that is, H-) can then form molecular bonds with neighboring gallium atoms”.
Perhaps the most surprising finding was in regards to the dissociation of hydrogen. Normally hydrogen requires high temperatures to dissociate by a thermal process. But gallium was found to induce hydrogen dissociation at much lower temperatures due to gallium transferring charge to the hydrogen molecule. As the transferred charge increases, the hydrogen bond orbital overlap decreases, eventually breaking the bond. The atoms then experience significant repulsion – a term called ‘Coulomb explosion’ (see Figure 3). Prof Ruoff notes that “dissociation and reassociation of hydrogen is an important aspect of the purification processes. As the ‘hydrogen economy’ is receiving considerable interest in Korea, more efficient ways of purifying hydrogen will become important. Our work suggests that further studies of these liquid metals as a route to purify hydrogen with much less energy, and with them also acting perhaps as a much cheaper catalyst, is an exciting path for more fundamental research!” He further noted that “by probing deeply into fundamental mechanisms of interactions between atoms in such new systems using state-of-the-art theoretical methods, new insights can be obtained, and we certainly hope that our modeling results will inspire experimentalists to probe such things as the charge states of the H and Ga atoms in this liquid metal system, and how H2 molecules might be dissociating in it, as well.”
This work is published as “Charge Transfer during the Dissociation of H2 and the Charge State of H Atoms in Liquid Gallium” in The Journal of Physical Chemistry C.
Available at https://doi.org/10.1021/acs.jpcc.9b06779
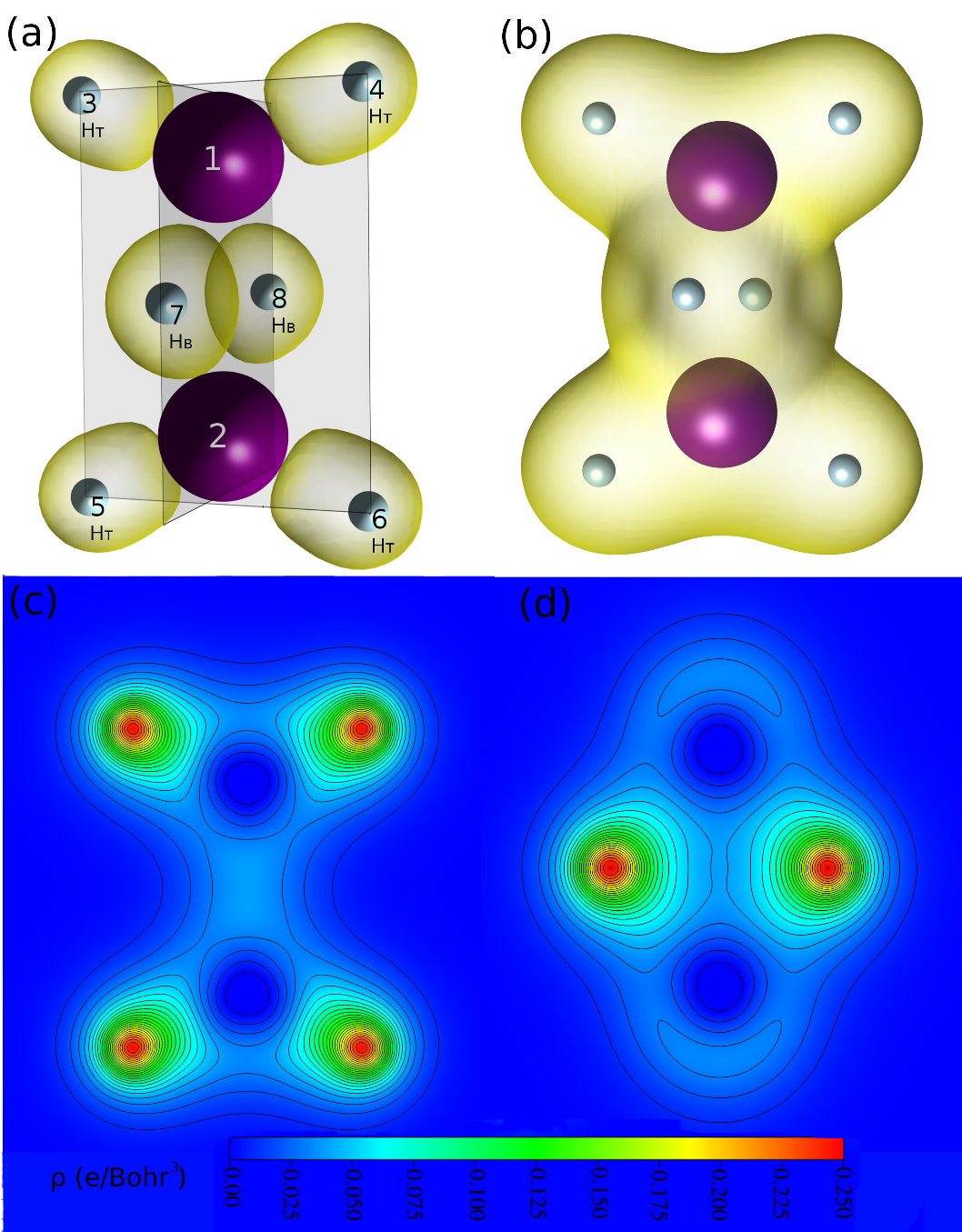
Figure 1. Electronic charge distribution in the digallane molecule (Ga2H6). (a) Schematic of digallane molecule with high electronic density regions shown. Atoms 1 and 2 are Ga, while 3 to 8 are H. HB and HT indicate the bridge and terminal hydrogen atoms, respectively. (b) Electron density. (c) Contour maps of valence electronic density in the plane that contains atoms 1 to 6, and (d) atoms 1, 2, 7, and 8, that shows that Ga atoms transfer charge to the H atoms.
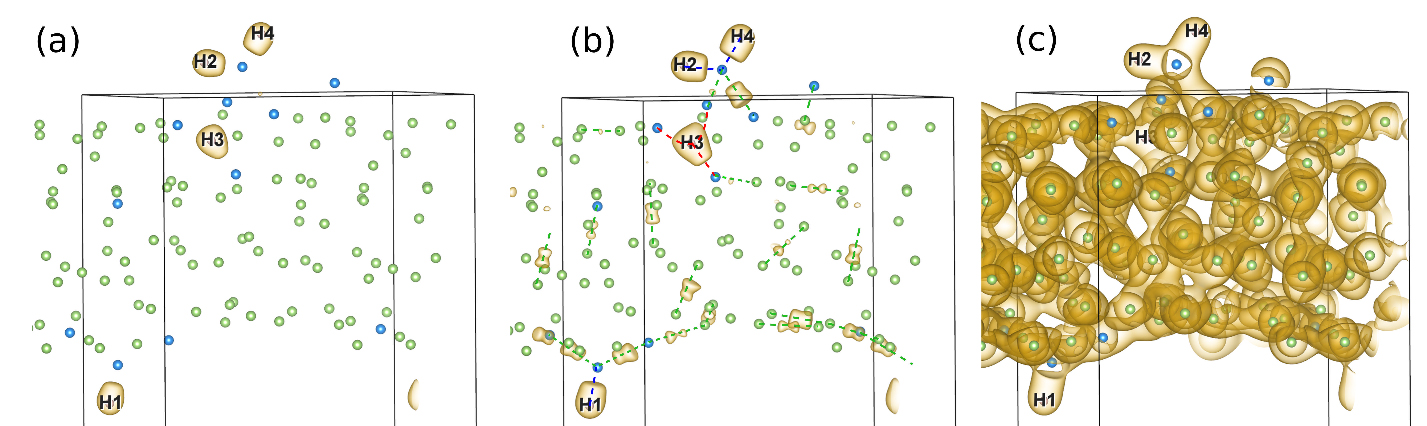
Figure 2. Electronic density of the Ga – H system from the Molecular Dynamics simulation. We can distinguish different types of bonds: (a) The points of highest electron charge density are around hydrogen atoms. (b) “Molecular-type” bonds are localized between hydrogen and some neigoring gallium atoms. (c) Metallic bonding is found throughout the liquid gallium system.
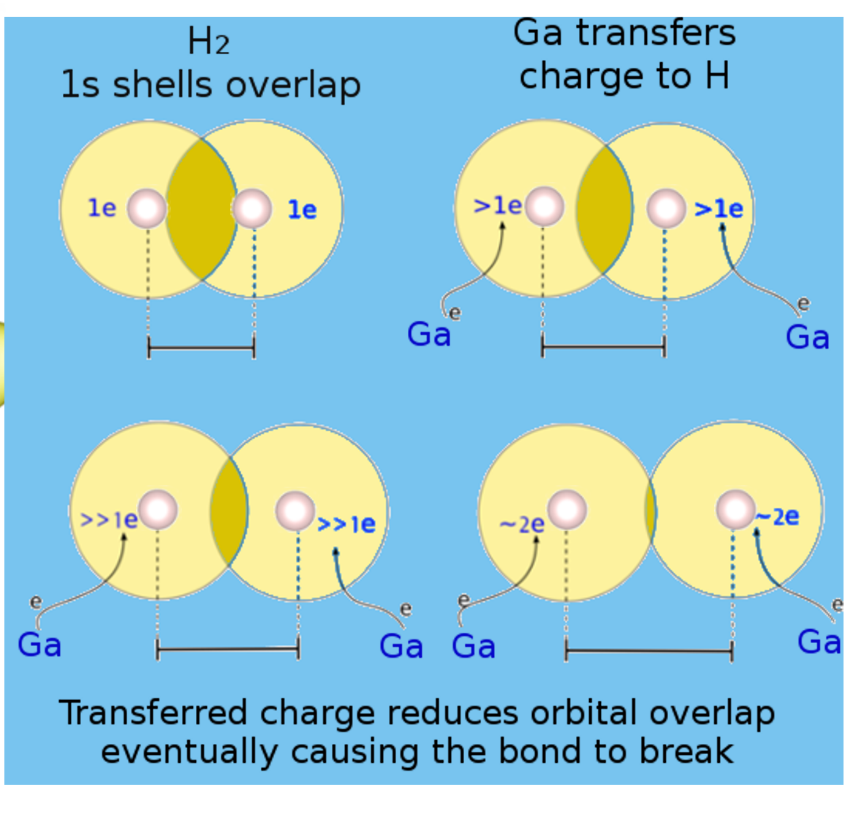
Figure 3. How the H2 molecule dissociated during this trajectory in liquid Ga. Schematic diagram explaining how charge transfer to the H2 molecule results in bond stretching and then breaking.
