UPDATES

the image editing credit goes to UNIST
The work by Distinguished Professor Rodney S. Ruoff has been published in Physical Review Letters. New study predicts, measures, and controls the temperature of large graphene bubbles with a single laser beam.
A recent study, affiliated with UNIST has measured and controlled the temperature of individual graphene bubbles with a single laser beam for the first time.
This study has been led by Distinguished Professor Rodney S. Ruoff (School of Natural Science) in collaboration with Distinguished Professor Feng Ding (School of Materials Science and Engineering) from the Center for Multidimensional Carbon Materials (CMCM) within the Institute for Basic Science (IBS) at UNIST. Their findings have been published in Physical Review Letters on May 3, 2018.
Through experiments, the team has newly identified that graphene bubbles are found to be more chemically reactive than flat graphene. This study demonstrates the potential of Raman Spectroscopy to provide valuable information on the physical properties, as well as on the chemical reactivity of graphene.
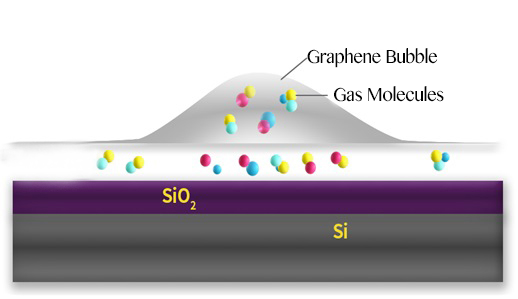
Schematic of a graphene bubble and trapped molecules between the SiO2 surface and graphene.
The highly elastic and flexible nature of graphene allows for the creation of stable large bubbles, in a more or less controlled fashion. The strain and curvature introduced by the bubbles is known to tune the electronic, chemical, and mechanical properties of this material. Generally, graphene bubbles are more reactive than flat graphene, so they might be more prone to be decorated with chemical groups. Bubbles might serve as tiny, closed reactors, and their curved surface could provide a lens effect. Understanding how temperature varies within bubbles is an important factor for several applications.
“If you think that chemical reactions could be carried out inside the bubble or on the surface of each graphene bubble, then changing the temperature distribution in a bubble will significantly influence reactions taking place,” says Dr. Yuan Huang, the first author of the study.
In this study, bubbles are formed at the interface between a graphene sheet and a silica (SiO2/Si) substrate it lies on. The SiO2 surface attracts some molecules that evaporate when heated, creating bubbles.
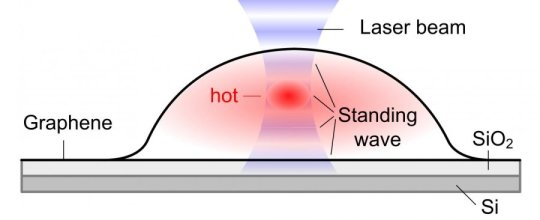
Bubbles form when molecules are trapped between the graphene sheet and the silica (SiO2/Si) substrate. The hottest spot is marked in red, which corresponds to the highest part of the bubble. l Image Credit: IBS
As also predicted by the theorists of the team, Dr. Xiao Wang and Professor Ding, the temperature oscillates with the bubble height. Although each bubble is only several micrometers in width and about one micrometer in height, the scientists could detect a variation in temperature, not only between the center and the edges, but also at different heights of the bubble.
When a graphene bubble is illuminated with a laser beam, incident and reflected rays overlap forming an optical standing wave on the surface. Increasing the laser power has the effect of selectively heating specific regions of the bubble, which correspond to the maximum interference of the standing optical wave. The team detected local changes in temperature within each bubble using Raman spectroscopy, a standard technique to measure graphene characteristics and morphology.
Among the researchers who partook in this study, above are UNIST-based researchers. From left are Xiao Wang, Distinguished Professor Fend Ding, Distinguished Professor Rodney Ruoff, and Ming Huang.
“Standing waves near surfaces have been ignored for a long time and have only rarely been observed in a direct manner. The results are surprising. The laser beam can efficiently heat the graphene, and we can determine the thermal conductivity in graphene bubbles from its temperature distribution,” explains Wolfgang Bacsa, one of the members of the team, and visiting scientist from CEMES-CNRS and University of Toulouse in France.
“These results confirm the high thermal conductivity of graphene previously measured, demonstrate the excellent adhesion around the perimeter of the graphene bubble, and provide new perspectives on how to heat graphene bubbles on specific locations,” concludes Distinguished Professor Ruoff, coauthor and director of CMCM. “The more we know about the physical properties of graphene bubbles, the more we might be able to make use of them in different ways.”
For example, an intriguing application could be the creation of graphene sheets with circular holes, like a ‘polka dot’ pattern. As overheating of the bubbles causes them to burst, the pores decorated with specific chemical groups could work as molecular selective filters. Graphene’s unique properties never cease to amaze.
Letizia Diamante
Science Writer and Visual Producer
T: +82-42-878-8260
E:letizia@ibs.re.kr
Journal Reference
Mandakini Biswal et al., “Raman Spectral Band Oscillations in Large Graphene Bubbles”, Physical Review Letters (2017). DOI: 10.1103/PhysRevLett.120.186104
*Article puclished on May 14, 2018
